The Science Behind Carbon vs Stainless Steel Knives
“Metallurgy - the science and technology of metals”.
- Merriam-Webster Dictionary
Alright Knife Nerds, it’s time to dive into our final segment on the Carbon vs Stainless Steel conversation from a technical level. At Town Cutler we are often asked what knife is better? This is a hard question to answer for there are similarities and differences on a molecular level, as well as, the maintenance of the blades. As noted throughout the first two blogs preference and lifestyle are important factors, so for this blog we get more scientific and thorough to illustrate the nature of the two steels and how we use them at Town Cutler. We discuss what elements make up our steel and how the differences could affect your knife. Additionally, we explore our heat treating process and how we sharpen our blades, regardless of the steels’ makeup. By learning some of the molecular differences we hope you gain a better understanding of the two steels and through explaining our heat treating and sharpening processes we hope you further appreciate the steps that a sheet of steel goes through to becoming a Town Cutler knife.
ELEMENTS AND STEEL COMPOSITION
All steels are alloys, or a combination of metals and other elements, with the most essential properties being iron and carbon. Other elements can be added to the steel and it is important to have a basic understanding of how the elements function within the overall steel structure.

This ingredient is essential to steel’s creation; our steel will have some amount of carbon. It is the most important hardening element, but as it is added it can reduce the toughness of the material. Carbon reduces the amount that the knife will wear over time. So, the amount of carbon in the blade tells you a lot about the quality of the steel.

A strong, hard magnetic silvery-gray metal, the chemical element of atomic number 26, much used as a material for construction and manufacturing, especially in the form of steel.


Strengthens the blade.

Hardens the blade. If added in high quantities it can increase brittleness.

Maintains the steel's strength at high temperatures.

Adds toughness and corrosion resistance.

Increases strength. Also, removes oxygen from the metal while it is being formed.

Increases machinability but decreases toughness.

Increases wear resistance.

Increases wear resistance and makes the blade harder.
Carbon and stainless steel knives both start from the same essential properties of iron and carbon but will exhibit different characteristics based on the elements added to the alloy. Elements such as manganese, chromium, cobalt, molybdenum and tungsten can be added to the alloy to contribute characteristics to the overall steel. While not in exact proportion sometimes when one element is added we are trading the effects of another. Each element has an effect on the steel, such as making it more resistant to corrosion or giving the blade more hardness which in turn makes it easier for the blade to retain an edge.
Town Cutler primarily uses 52100 carbon steel and AEB-L stainless steel for our blades. We select these steels because we believe these grades of steel provide the most strength and durability for our knifemaking and for our finished product. By looking at the specific elemental makeup of these steels, we can start to understand more about their key characteristics and how they will behave.
Town Cutler uses 52100 carbon steel made of
Carbon .98%-1.1%, Chromium 1.3%-1.6%, Manganese .25%-45%, Sulfur .025% and Silicon .15%-.35%
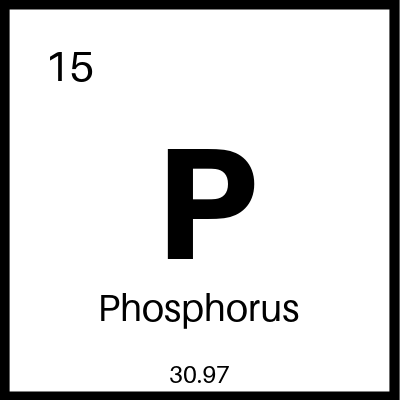
Town Cutler uses AEB-L stainless steel made of
Carbon .67%, Chromium 13%. Silicon .40%, Manganese .60%, Phosphorus .025% and Sulfur .015%
52100 carbon steel is strong yet can be easily ground into a sharp blade. This grade of steel has very little chromium, which means it can attain a more precise edge and has excellent structural memory and flexibility. However, the lack of chromium (1.3%-1.6%) causes it to be more reactive to moisture, meaning that the blade has little resistance to rusting. Most carbon blades are susceptible to iron oxidation and the negative effects of acidic foods, but with proper care the 52100 fares much better.
We use AEB-L stainless steel for the majority of our blades. It is a very durable steel that is tough and because of its elemental properties, is also easy to work with and can be sharpened to a razor sharp edge. In fact, AEB-L steel was originally developed to be a razor steel and have great edge retention. As opposed to our 52100 carbon steel, AEB-L has significantly more chromium, making it highly resistant to corrosion. When we look at the elemental composition of AEB-L steel, we can see that the addition of specific elements, and in which specific amounts, leads to a steel that is extremely strong and highly resistant to corrosion, but with great edge retention.
HEAT TREATING
Now that we have established what types of steel we use and the importance of the specific elements that make up the steel, we can change the focus to the heat treating process, which is how our steel becomes what it needs to be. When steel comes to the Town Cutler warehouse it is flexible and malleable, regardless of whether it is carbon or stainless. In this state it is soft and will not hold an edge, therefore it must undergo heat treatment. Heat treating is the hardening process of steel and is a controlled process involving building and releasing tension in the steel through multiple steps of heating and cooling the material at precise times and temperatures. To determine the hardness of steel, Town Cutler uses the Rockwell scale, which is acknowledged as one of the most widely accepted and accurate methods to determine a metal's hardness. Before undergoing heat treatment, the steel as it arrives at the warehouse will register at below 20 Rockwell (HRC). For perspective, most culinary knives will range from 55-64 HRC. It is the very important process of heat treating that transforms the steel, so that once heat treated properly, our steel will test between 61-63 on the Rockwell scale.

52100 Heat Treat Chart
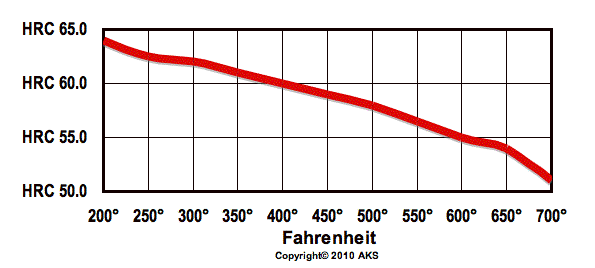
52100 Tempering Chart
The process of heat treating starts with the soft steel being put into an oven with the heat ranging from 1500-2000 °F. As the temperature increases the steel’s grain structure gets smaller and the molecules move around rapidly. If you’ve ever seen a blade coming out of a heat treating oven that glows bright orange, that is caused by the molecules rapidly moving around. Within every metal there are grain structures surrounded by grain boundaries and it is within these boundaries that the grain structures are strengthened by the heat treating process. After this initial change in the grain structure of the steel, it has become austenite. Austenite is when iron steel is heated so that the grain structure changes and the molecules are at their finest, meaning they are tighter and smaller, providing more strength. Austenite is what we want to achieve during this stage of heat treating and if the steel was to stay too long in the oven or get too hot, the molecules would balloon and the steel may not reach the hardness or durability that it needs to be. This is why time and temperature are the most important factors in this process. Immediately after being in the oven the steel is quenched (cooled down) in oil. It is during this change that the steel becomes strengthened and the grain structure further transforms into what is called martensite, which is when the steel is no longer austenite and due to the quenching some carbon properties have dissolved.
Once cooled to room temperature the steel has the strength we want, however it is slightly too hard for our needs at 64-68 HRC, at that hardness it is too brittle to shape and fragile enough that if dropped it could shatter like glass. In order to combat the brittleness of the steel, within an hour after quenching, it is placed back into the oven between 300-500°F. By placing the steel back into the oven after quenching, the steel can now relax dropping to the preferred 61-63 HRC, while also locking in the strength and toughness we want. The process of placing a quenched steel into a heat controlled oven and vice versa is known as tempering. Within this process we create a spring steel quality in our knives, spring steel memory refers to the memory of a knife.
After the steel has gone through the multi-cycle process of heating, quenching, and cooling, it is now durable enough to be shaped into a blade while keeping the integrity and strength of the steel. Because of the relaxed tensions within the grain structure the knife now has its own memory allowing it to stay hard but still retain an edge. One can feel this memory with their finished knives, for example when you hit a bone or a seed your knife is less likely to chip, crack or suffer any major structural damage. If a knife blade is heat treated properly then it is capable of being slightly bendable to allow for flex while maintaining its original shape. This quality also allows the steel to maintain better edge retention.
All of this means high heat changes the physical properties in metal, and can do so even after a knife is complete. For example, one should never blow torch a knife. By heating the knife, the grain structure changes and the heat will soften the steel, making the knife lack edge retention capabilities and making the blade weaker than before. How high heat changes the physical properties of steel is not only extremely important during the heat treating process, but remains so during the lifetime of the steel.
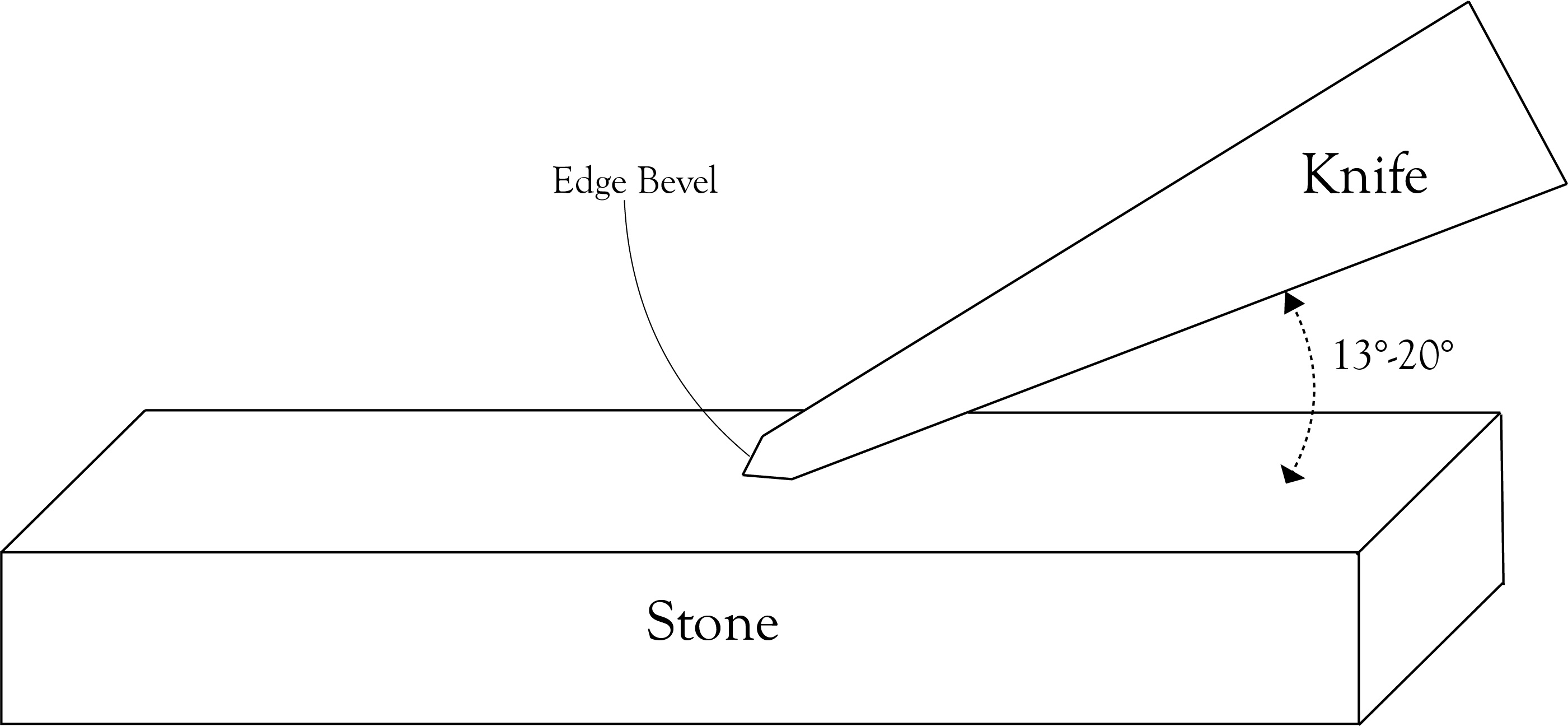
BEVELING AND EDGE GEOMETRY
At this point It is important for us to highlight our sharpening methods. Town Cutler grinds both steel knives down to .10 (10,000 of an inch) at the edge of the blade during our beveling. Due to the components of the steel, 52100 and AEB-L can be thinned down and still retain a sharp edge while maintaining their durability. Our knives are sharpened at a 13-15° angle because once the toughness of our steel is met (61-63 HRC) it can withstand a lower sharpening angle. The reason for sharpening at those specific angles is because we know that our steel is hard enough and can maintain its memory. A blade with a lower Rockwell scale grade, such as 60 HRC, would be sharpened at a 20° angle, this is not to say it is a bad knife, we are just explaining that at Town Cutler we only want our steel to be at our specified hardness to ensure it can be sharpened to a finer edge, which is necessary if you want the most efficiency and precision in a knife.
It is relevant to mention that Town Cutler uses Japanese whetstones to sharpen our knives so the blades stay cool, as opposed to belt sharpening which creates a lot of friction and heat, which could change the grain structure of the steel. Similarly to how using a blow torch on your knife can change the grain structure and steel qualities, a belt sharpener could heat your blade, and when heated over and over again there is a possibility that it could make the knife’s steel structure weaker, leading it to become duller faster, hold less memory, and not be able to achieve as sharp on an edge. By using the steel that we do and following proper heat treating, this all enables us to sharpen our blades to perfection, and for our customers, as a chef in any capacity, to ensure that your knife is performing at its best.
Town Cutler uses 52100 carbon and AEB-L stainless steel for our knives because we feel that these grades of metal make the best blades we can produce. The differences between the two exists on a molecular level, but are also visible to the naked eye. For example when you pass by a knife with a darker patina on the blade you can now say to yourself, “that is probably a carbon steel knife, it’s the lack of chromium which makes it more susceptible to patinated and rusting.” But whether selecting a carbon or stainless steel knife it is the caliber of the steel, the heat treating process, and the edge geometries of the blade are the most important factors in the overall outcome of the knife. As discussed in our previous blogs, the use of either carbon or stainless steel is mainly a preference by the owner. A good quality knife that is sharp will cut regardless of being carbon or stainless steel. The important thing is that the knife is sharp and performs at its highest potential.
- Thanks for reading, Stay Sharp!